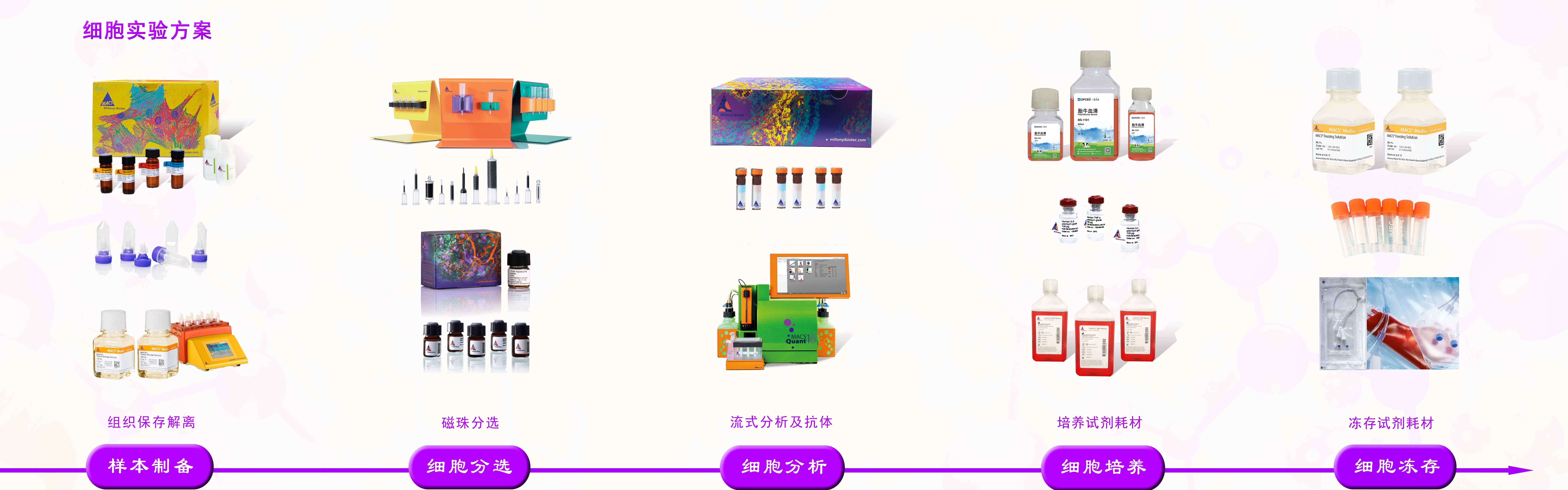
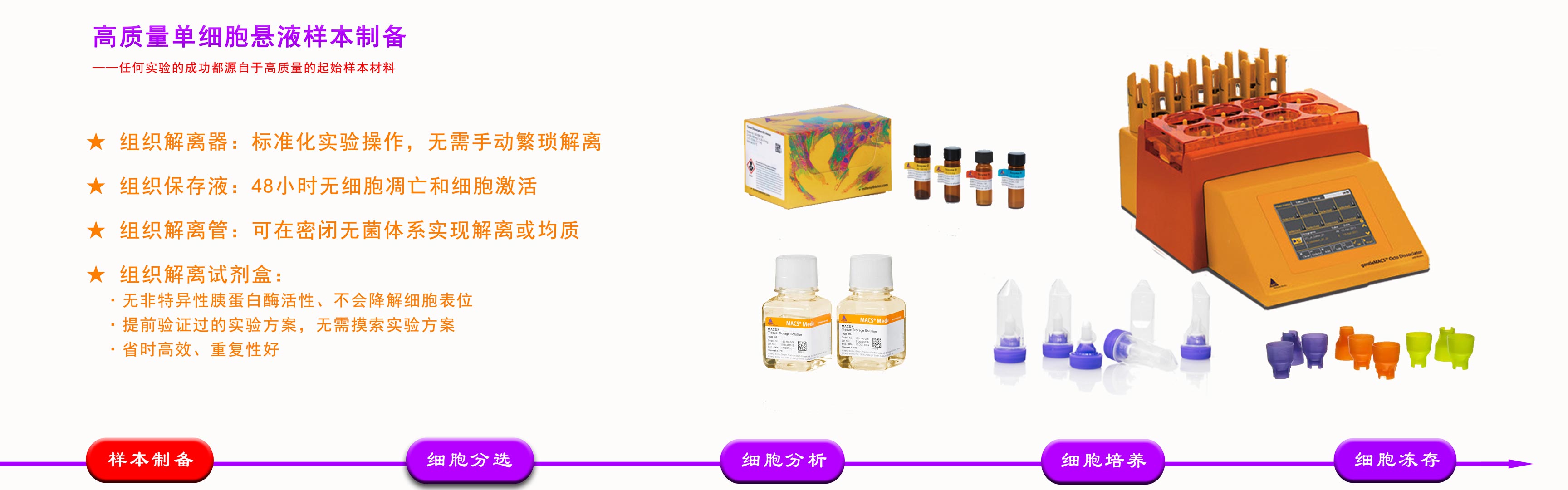
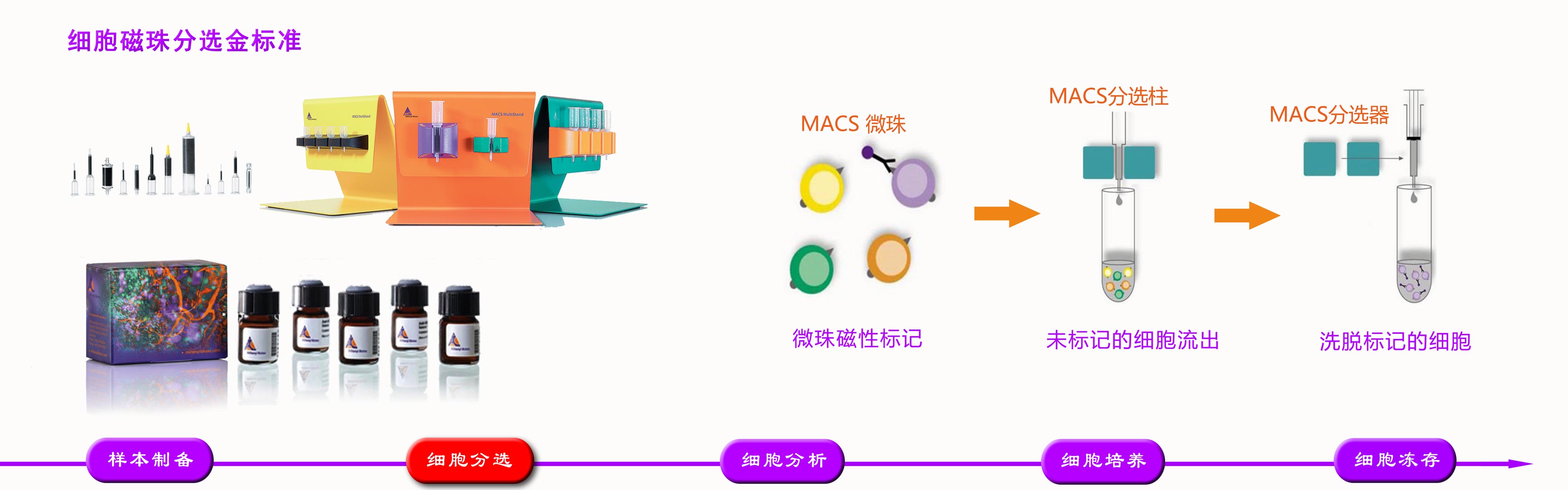
品牌:Miltenyi 货号:170-076-309 规格:1000mL
产品介绍
TexMACS Medium是一种优化的无血清细胞培养基,用于培养和扩增人和小鼠T细胞和调节性T细胞。这种完整的培养基可以在人类和小鼠细胞培养中重复应用。它不含动物源性成分。
TexMACS GMP培养基不含血清和异种成分。
•优化配方含有葡萄糖和稳定的谷氨酰胺(l-丙烯酰- l-谷氨酰胺)
•QC功能测试每批
产品优点
1、无血清、无动物源性成分;
2、 高性能和高活性;
3、 高一致性和稳定性;
4、灵活的包装形式。
产品应用
T细胞扩增培养
Publications
1.Schwarze, L. I. et al. (2021) Automated production of CCR5-negative CD4+-T cells in a GMP-compatible, clinical scale for treatment of HIV-positive patients. Gene Ther. 28 (9): 572-587
2.Fraser, A. R. et al. (2017) Development, functional characterization and validation of methodology for GMP-compliant manufacture of phagocytic macrophages: A novel cellular therapeutic for liver cirrhosis. Cytotherapy 19 (9): 1113-1124
3.Tischer, S. et al. (2014) Rapid generation of clinical-grade antiviral T cells: selection of suitable T-cell donors and GMP-compliant manufacturing of antiviral T cells. J. Transl. Med. 12: 336
4.Fernández, L. et al. (2019) GMP-Compliant Manufacturing of NKG2D CAR Memory T Cells Using CliniMACS Prodigy. Front Immunol 10 (10): 2361
5.Priesner, C. et al. (2016) Comparative analysis of clinical-scale IFN-γ-positive T cell enrichment using partially and fully integrated platforms. Front Immunol 7: 393
6.Fraser, H. et al. (2018) A Rapamycin-Based GMP-Compatible Process for the Isolation and Expansion of Regulatory T Cells for Clinical Trials. Mol Ther Methods Clin Dev. 31649672 (8): 198-209
7.Bernaldo-de-Quirós, E. et al. (2022) A Novel GMP Protocol to Produce High-Quality Treg Cells From the Pediatric Thymic Tissue to Be Employed as Cellular Therapy. Front Immunol 16 (13): 893576
8.Glienke, W. et al. (2022) GMP-Compliant Manufacturing of TRUCKs: CAR T Cells targeting GD2 and Releasing Inducible IL-18. Front Immunol 24 (13): 839783
9.Cooper, R. S. et al. (2021) Rapid GMP-Compliant Expansion of SARS-CoV-2-Specific T Cells From Convalescent Donors for Use as an Allogeneic Cell Therapy for COVID-19. Front Immunol 11: 598402
10.Lavazza, C. et al. (2022) Process development and validation of expanded regulatory T cells for prospective applications: an example of manufacturing a personalized advanced therapy medicinal product. J. Transl. Med. 20 (1): 14
11.Joedicke, J. J. et al. (2021) Accelerating clinical-scale production of BCMA CAR T cells with defined maturation stages. Mol Ther Methods Clin Dev. 25 (24): 181-198